
Where science
meets heart
Thalassemia Resources
for Healthcare Providers
Clinical trials
Thalassemia Clinical Program
Agios is committed to developing innovative treatment options that make a meaningful difference in patients’ lives and fundamentally change the way rare and genetically defined diseases are treated. We are leveraging our leadership in PK activation to explore an investigational, oral treatment for thalassemia. More information about our active trials is below.
Disease education
View short video on the thalassemia mechanism of disease

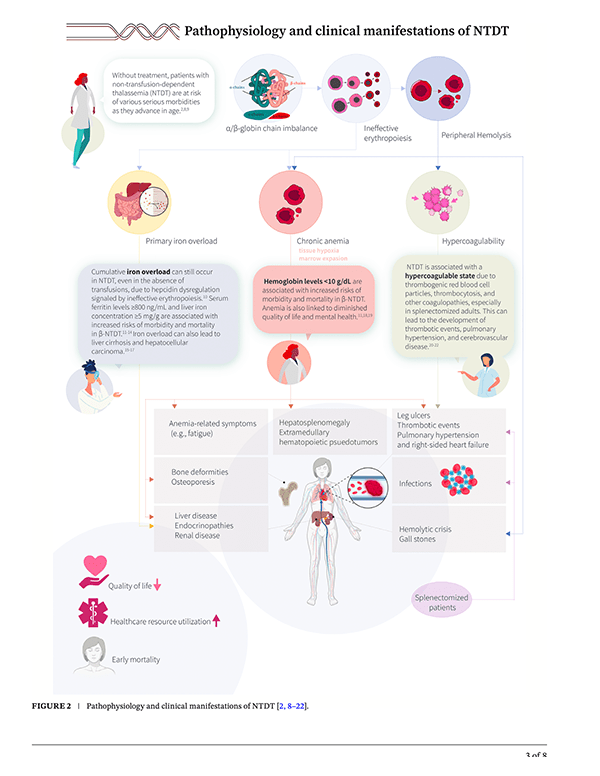
Non-Transfusion-Dependent Thalassemia: An Image Gallery Worth a Thousand Words
A pictorial manuscript published by Dr. Musallam, et al., in the American Journal of Hematology
Musallam KM, Sheth S, Coates TD, et al. Non-Transfusion-Dependent Thalassemia: An Image Gallery Worth a Thousand Words. Am J Hematol. 2025;100(4):687–694.
creativecommons.org/licenses/by/4.0/

Beta-Thalassemia Fast Facts Patient Booklet
For healthcare providers to use with patients
Download PDF
Italian / Spanish / Arabic / French / Hindi / Bengali / Urdu / Vietnamese / Chinese

Alpha-Thalassemia Fast Facts Patient Booklet
For healthcare providers to use with patients
Download PDF
Italian / Spanish / Arabic / French / Hindi / Thai / Bengali / Urdu / Vietnamese / Chinese

Fast Facts Information Sheet: Understanding Alpha Thalassemia
For healthcare providers to use with patients
News
Thalassemia Newsletter
“Thal Pals: The Alpha Beta Revolution” Podcast
Thal Pals: The Alpha Beta Revolution is a podcast intended for patients, caregivers, providers and the greater community of people who are impacted by thalassemia. Each episode strives to provide listeners with critical education, the latest scientific updates and voices from the thalassemia community. The podcast is sponsored by Agios Pharmaceuticals, Inc. The program is intended for informational and educational purposes only and is not intended as medical advice. Host and guests featured in each episode have been compensated for their time.
Scientific resources
PK Deficiency

Pyruvate kinase (PK) deficiency is a rare, hereditary, chronic hemolytic anemia caused by mutations in the PKLR gene encoding the enzyme pyruvate kinase, which is critical for maintaining red blood cell (RBC) energy levels and normal RBC life span. Defects in pyruvate kinase lead to premature destruction of erythrocytes which manifest clinically as anemia and serious complications including gallstones, pulmonary hypertension, thrombosis, osteopenia, osteoporosis, and iron overload. Currently, Agios is studying the long-term safety and efficacy of PK activation in adults with PK deficiency and has expanded the clinical trial program to study PK activation in pediatric patients with PK deficiency from 1-17 years of age.
Sickle Cell Disease

Sickle cell disease (SCD), a monogenetic disease of hemoglobin, affects millions across the globe. The resulting sickled red blood cell (RBC) leads to a host of complications including anemia, hemolysis, and episodes of acute pain, among others. People with SCD continue to have needs not met by current therapies. At Agios, we are studying how PK activation could potentially benefit people with SCD. PK activation reduces 2,3-diphosphoglycerate (2,3-DPG) levels in RBCs which increases hemoglobin oxygen affinity, reducing hemoglobin S polymerization and may inhibit the sickling process. At the same time, increasing adenosine triphosphate (ATP) enhances the energy metabolism of the RBC which may lead to improved membrane integrity and RBC health. Currently, Agios is studying PK activation in adults with SCD in ongoing clinical trials to assess both the effect on anemia and vaso-occlusive events.
For medical information queries, please contact us at the phone numbers or email addresses below.
Report an adverse event or product complaint anytime.
Outside the U.S.:
All countries: ex-usmedinfo@agios.com
France: +33 801841438
Germany: 08001090777
Italy: +39 800596326
Spain: +34 900990230
Monday – Friday
9:00 am – 5:00 pm Local standard time
This site is intended for healthcare professionals; all materials available are for scientific informational purposes only.
Resources available may include educational and information materials relating to Agios’ clinical pipeline, investigational products, therapeutic areas of interest, approved medicines, congress materials, and publications. This site may include information that has not been approved by the US Food and Drug Administration. There is no guarantee that investigational products will receive health authority approvals or become commercially available in any country for the uses under investigation. For approved product full prescribing information, including indications, contraindications, warnings, precautions, and adverse events, please refer to approved product labeling.
MIT-ALL-0179 11/22











